Successful Creation of a Candidate Compound for the Treatment of Intractable Pain by Chemical Modification of Pimozide, a Typical Antipsychotic
Summary
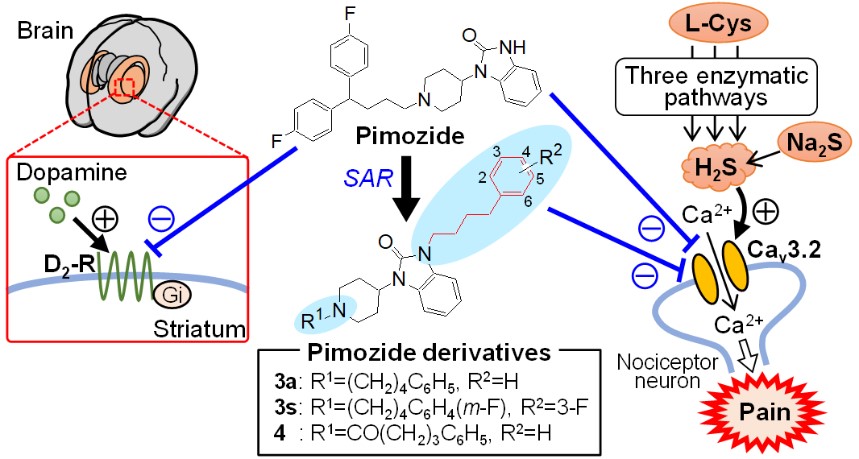
T-type Ca2+ channels (T-channels), especially Cav3.2, have attracted attention as therapeutic targets for various diseases including intractable pain. In collaboration with Prof. Atsushi Kawabata, Faculty of Pharmaceutical Sciences, Kindai University, Prof. Naoki Toyooka and Assistant Prof. Takuya Okada have discovered that the typical antipsychotic drug, pimozide, has a strong inhibitory activity on T-channels. By applying appropriate chemical modifications to pimozide, they succeeded in synthesizing new pimozide derivatives 3a, 3s, and 4, which have T-channel inhibitory activity comparable to that of pimozide and little binding affinity to dopamine D2 receptor (D2R). These novel pimozide derivatives potently inhibited T-channel-dependent somatic and visceral pain in mice, but did not cause any motor dysfunction caused by D2R blockade, such as catalepsy. The novel pimozide derivatives 3a, 3s, and 4 found in this study are extremely promising compounds for the treatment of intractable pain.
Background and Results
Among the T-type Ca2+ channel (T-channel) family members, Cav3.2 is abundantly expressed in primary sensory nerves and contributes to the development of neuropathic pain and visceral pain. Although some existing drugs have T-channel inhibitory activity, their application to the treatment of pain has not been reported to date. Pimozide, a typical antipsychotic, has potent T-channel inhibitory activity in addition to dopamine D2 receptor (D2R) blocking activity as its main action. In this study, we attempted to develop a new T-channel inhibitor that does not exhibit D2R-blocking activity by conducting structural expansion of pimozide with the aim of developing a new therapeutic agent for intractable pain.
After synthesizing a total of 32 pimozide derivatives, we found that the new pimozide derivatives 3a (IC50 value 0.46 µM), 3s (IC50 value 0.09 µM), and 4 (IC50 value 0.15 µM) have T-channel inhibitory activity comparable to that of pimozide (IC50 value 0.16 µM) but with little binding affinity to the D2R. We found that the introduction of the phenylbutyl moiety into the benzoimidazole ring system of pimozide contributed significantly to the decrease in D2R binding affinity. In particular, 3a and 3s potently suppressed Cav3.2-dependent somatic and visceral pain in mice and did not cause motor dysfunction attributable to D2R blockade, including catalepsy and neuromuscular dyscoordination.
These results suggest that the synthesized pimozide derivatives, especially 3a, 3s, and 4, are extremely promising candidate compounds for the treatment of intractable pain and itching, including neuropathic pain and visceral pain.
Graphic abstract
Original article information
Journal
European Journal of Medicinal Chemistry
Title
Discovery of Pimozide Derivatives as Novel T-type Calcium Channel Inhibitors with Little Binding Affinity to Dopamine D2 Receptors for Treatment of Somatic and Visceral Pain
Authors
Yoshihito Kasanami, Chihiro Ishikawa, Takahiro Kino, Momoka Chonan, Naoki Toyooka, Yasuhiro Takashima, Yuriko Iba, Fumiko Sekiguchi, Maho Tsubota, Tsuyako Ohkubo, Shigeru Yoshida, Atsushi Kawase, Takuya Okada, Atsufumi Kawabata

